렌티바이러스 (Lentivirus Packaging)
재조합 렌티바이러스(lentivirus)는 포유류 세포로 효율적인 유전자 전달을 위해 가장 흔히 사용되는 바이러스 벡터입니다. 호스트 세포에서 외래 유전자의 일시적이며 episomal한 발현만 가능한 plasmid DNA 벡터와 달리, 렌티바이러스 벡터는 호스트 세포의 유전체 내로 외래 유전자를 결합함으로써 영구적으로 발현되도록 할 수 있습니다. 렌티바이러스는 생체내 유전자 전달에도 효율적입니다.
VectorBuilder에서는 우수한 품질의 렌티바이러스 패키징 서비스를 제공합니다. 특히 당사는 벡터 제작 서비스에 사용되는 3세대 렌티바이러스 벡터 시스템을 위한 titer, 순도, 생존력 및 일관성 측면에서 재조합 렌티바이러스 생산 과정을 크게 개선한 자체의 기술과 시약들을 개발해왔습니다. 그 결과 렌티바이러스 벡터 클로닝과 패키징에 대한 고객의 요구를 만족시킬 수 있는 서비스를 제공함으로써 당사에 반복적으로 제작을 의뢰하는 고객들이 점점 많아지고 있습니다. 연구용 렌티바이러스 이외에도 임상 응용을 위한 GMP-grade의 렌티바이러스를 생산하는 서비스도 제공하고 있습니다.
제공되는 렌티바이러스의 유형
- VSV-G pseudotype된 3세대 렌티바이러스 (기본 바이러스 유형)
- VSV-G pseudotype된 2세대 렌티바이러스
- Coronavirus spike (S) proteins과 같은 다른 envelope 단백질이 pseudotype된 렌티바이러스
- Envelope 단백질이 없는 렌티바이러스 (transduction시에 negative control로 사용될 수 있습니다)
- Integrase가 없는 렌티바이러스 (IDLV)
서비스 세부 사항
가격 및 소요 시간 Price Match
| Scale | Application | Typical Titer | Minimum Titer | Volume | Price (USD) | Turnaround |
|---|---|---|---|---|---|---|
| Mini | Cell culture | >2x10 8 TU/ml | >10 8 TU/ml | 100 ul (4x25 ul) | $199 |
6-12 days
|
| Pilot | >4x10 8 TU/ml | 250 ul (10x25 ul) | $449 | |||
| Medium | >3x10 8TU/ml | 1 ml (10x100 ul) | $649 | |||
| Large | >2x10 9TU/ml | >10 9TU/ml | 1 ml (10x100 ul) | $1,099 | ||
| Ultra-purified medium | Cell culture & in vivo | >2x10 9TU/ml | >10 9TU/ml | 500 ul (10x50 ul) | $1,399 | |
| Ultra-purified large | 1 ml (10x100 ul) | $1,699 |
주:
1. 위의 표는 VSV-G pseudotype된 2세대 또는 3세대 렌티바이러스의 경우에 적용됩니다 (integrase-deficient lentivirus (IDLV) 포함).
2. Coronavirus S protein으로 pseudotype된 렌티바이러스에 대한 자세한 서비스 내용을 원하시면 여기를 눌러주시기 바랍니다.
3. TU = Transduction units (also known as infectious units).
결과물
| For non-ultra-purified scales | For ultra-purified scales |
|---|---|
| Your custom lentivirus | Your custom lentivirus |
|
Free:control virus
|
Add-on purchase (optional):ultra-purified control virus
|
|
Free:Polybrene (5 mg/ml, 200 ul) |
Free:Polybrene (5 mg/ml, 200 ul) |
Control 바이러스
Control 바이러스는 맞춤형 바이러스의 생물학적 용도와 일치하도록 디자인되어, 렌티바이러스 transduction을 테스트하는 데 사용됩니다. 예를 들어 맞춤형 바이러스가 유전자를 과발현하는 경우, 제공되는 control 바이러스는 EGFP control 렌티바이러스 (EGFP 과발현 렌티바이러스)이고, 맞춤형 바이러스가 유전자에 대한 shRNA를 발현하는 것이면 제공된 control 바이러스는 scramble shRNA를 발현하는 것입니다. Control 바이러스에 대한 자세한 정보는 다음과 같습니다.
| 벡터 시스템 | Control 바이러스 벡터 이름 | Control 바이러스 벡터 ID |
|---|---|---|
| 렌티바이러스 유전자 발현 시스템 | pLV[Exp]-EGFP/Puro-EF1A>mCherry | VB010000-9298rtf |
| 렌티바이러스 CRISPR 시스템 | pLV[Exp]-EGFP/Puro-EF1A>mCherry | VB010000-9298rtf |
| U6 기반의 shRNA knockdown 렌티바이러스 시스템 | pLV[shRNA]-EGFP/Puro-U6>Scramble_shRNA | VB010000-0009mxc |
| mir-30 기반의 shRNA knockdown 렌티바이러스 시스템 | pLV[miR30]-EGFP/Puro-EF1A>mCherry:Scramble_miR30-shRNA | VB010000-9387zjj |
| IPTG에 의해 유도되는 shRNA knockdown 렌티바이러스 시스템 | pLV[shRNA]-LacI/Puro-U6/2xLacO>Scramble_shRNA | VB010000-9348prq |
배송 및 보관
렌티바이러스는 HBSS buffer에 녹인 후 얼려서 드라이아이스와 함께 배송됩니다. 수령 즉시 장기보관을 위해 (최소 6개월 이상 안정) -80℃에서 보관해야 하며, 1주일 이내에 사용시에는 -20℃에 보관하여야 합니다. 렌티바이러스의 유통기한은 약 1년입니다. 렌티바이러스의 반복적인 동결-융해는 심각한 titer 감소를 초래할 수 있기 때문에 피하여야 합니다.
기술적인 정보
렌티바이러스 생산 및 QC
3세대 렌티바이러스 패키징을 위하여 목적 유전자(GOI)를 운반하는 transfer plasmid는 당사 소유의 VSV-G를 인코딩하는 envelope plasmid와 Gag/Pol 및 Rev를 인코딩하는 패키징 plasmid와 함께 HEK293T 패키징 세포에 co-transfection 됩니다. 48시간의 배양 후 상청액을 채취하고 원심분리하여 세포 이물질을 제거한 후 여과합니다. 렌티바이러스 입자는 이후 PEG에 의하여 농축됩니다. Ultra-purified 렌티바이러스의 경우 (in vivo grade), 바이러스 입자를 sucrose cushion ultracentrifugation으로 추가 정제하고 ultrafiltration으로 농축합니다. 당사는 p24 ELISA 방법을 사용하여 lentivirus titer를 측정합니다.
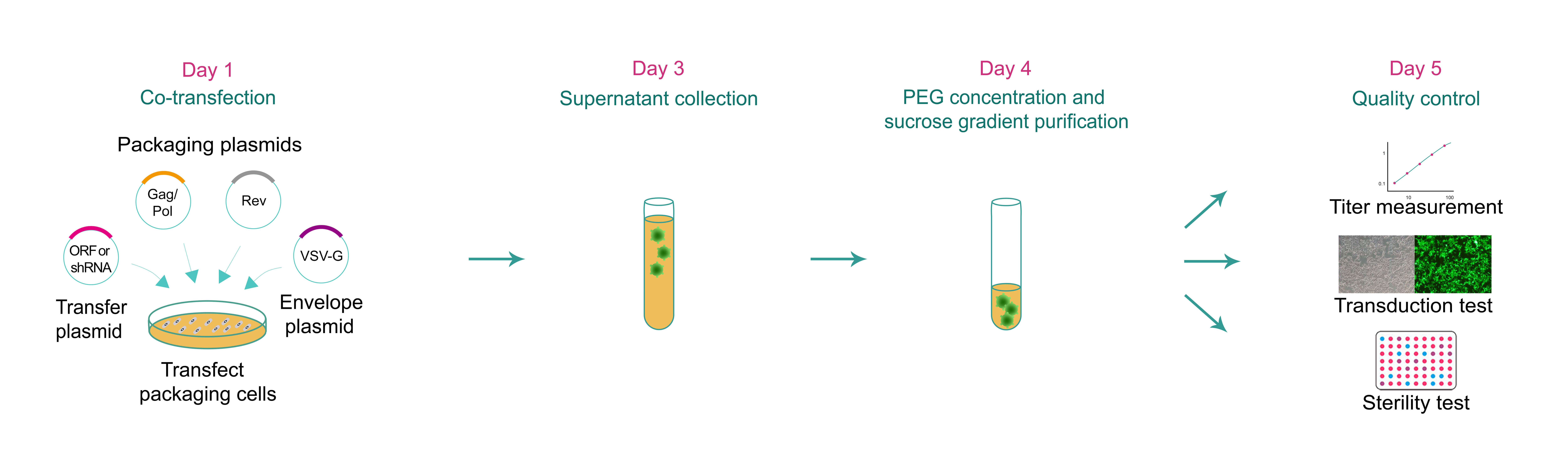
VectorBuilder에서 생산되는 각 재조합 렌티바이러스에 대한 품질관리에는 titer 측정, 박테리아 및 fungi에 대한 무균 테스트, mycoplasma 검출 등이 있습니다. Transfer 벡터가 형광 단백질을 인코딩하는 경우, 해당 형광을 검출하기 위해 transduction 테스트를 수행합니다. Transfer 벡터가 항생제 선별 marker를 인코딩하면, transduction 테스트를 수행한 다음 해당 항생제 선별을 수행합니다. 추가로 ultracentrifuge에 의해 정제된 렌티바이러스의 경우, 일반적으로 endotoxin 수준를 체크하기 위해서 endotoxin 분석을 수행합니다. Endotoxin 테스트 결과를 COA에 포함하려면 추가 비용이 필요합니다. 추가 QC 서비스는 별도 요청시 제공될 수 있습니다.
주문 방법
고객이 제공하는 벡터
고객이 제공하는 벡터가 필요한 경우 시료 제출 가이드라인에 따라서 벡터를 보내주시기 바랍니다. 배송 지연이나 시료 손상을 방지하기 위해 당사의 가이드라인을 엄격히 따라 주시기 바랍니다. 모든 고객이 제공하는 시료는 VectorBuilder에 의해 의무적으로 QC를 거치며 각 품목에 대해 $100의 추가 요금이 부과될 수 있습니다. 고객이 제공한 시료가 QC를 통과할 때까지는 생산을 시작할 수 없음을 유의하시기 바랍니다.
자료
문서 자료
사용 설명서 물질안전보건자료 (MSDS) 시험성적서 (COA) 브로셔자주 묻는 질문
| 렌티바이러스 | MMLV | 아데노바이러스 | AAV | |
|---|---|---|---|---|
| Tropism (친화성) | 넓음 | 넓음 | 어떤 세포들에는 비효율적임 | 바이러스 serotype (혈청형)에 따라서 다름 |
| 분열을 하지 않는 세포에 감염이 가능한가? | Yes | No | Yes | Yes |
| 호스트 유전체에 안정한 결합을 하는가 아니면 단기적인가? | Stable integration | Stable integration | Transient, episomal | Transient, episomal |
| 최대 titer | High | Moderate | Very high | High |
| 맞춤형 promoter | Yes | No | Yes | Yes |
| 주요 용도 | Cell culture and in vivo | Cell culture and in vivo | In vivo | In vivo |
| In vivo에서의 면역반응 | Low | Low | High | Very low |
VectorBuilder에서는 p24 ELISA에 의하여 렌티바이러스의 titer를 결정합니다. 렌티바이러스가 포함된 세포 배양 상청액에 있는 HIV-1 p24 core 단백질을 정량하기 위하여 sandwich immunoassay를 이용합니다. 먼저 렌티바이러스 샘플 내의 p24와 결합하기 위한 anti-HIV-1 p24 capture antibody로 코팅된 microtiter plate에 상청액을 넣습니다. 그 다음에 biotinylated anti-p24 secondary antibody를 넣으면 p24 antibody에 결합된 p24에 결합을 합니다. Streptavidin-HRP conjugate를 그 다음에 넣으면 streptavidin과 biotin이 결합하며, 최종적으로 HRP와 substrate의 반응에 의하여 발색하게 됩니다. 이 때 나타나는 색의 강도는 렌티바이러스 샘플 내에 존재하는 p24의 양에 비례하며, spectrophotometer를 사용하여 재조합 HIV-1 p24 standard curve와 비교함으로써 정확한 양을 결정할 수 있습니다. p24 수치로부터 최종적으로 렌티바이러스 샘플 내의 바이러스 titer를 얻게 됩니다.
당사에 의하여 보장되는 titer는 패키징 되는 Δ5’ LTR에서 ΔU3/3’ LTR까지의 영역이 렌티바이러스 cargo limit (9.2 kb) 이하일 때 적용됩니다. 그 이상의 경우 여전히 벡터를 바이러스로 패키징할 수는 있지만 titer는 감소할 수 있습니다. 또한 다음과 같은 벡터들의 경우 titer를 보장해드릴 수 없습니다.
- Proapoptotic 유전자와 같이 숙주 세포에 대한 독성을 나타낼 수 있는 유전자들, 세포막 단백질과 같이 세포들이 응집하게 만들어서 세포나 바이러스의 integrity를 손상시킬 수 있는 유전자들과 같이 패키징 과정에 유해한 작용을 할 수 있는 서열들이 포함되어 있거나, 반복 서열 또는 GC 함량이 매우 높아서 재배열이나 2차 구조를 만들 가능성이 높은 서열을 포함하는 벡터들.
- 고객이 제공한 plasmid에 패키징 효율에 불확실성을 제공할 수 있는 공개되지 않은 서열이나 일반적이지 않은 렌티바이러스 기능을 가진 요소 (예를 들어 LTR) 를 포함하는 경우.
예상 소요 시간은 제조 시작부터 완료까지의 시간을 의미합니다. 고객이 제공한 시료 (template DNA나 바이러스 벡터 등)의 운송 시간과 QC, 최종 결과물을 고객에게 배송하기 위한 운송 시간은 포함되지 않습니다.