바이러스 제조

VectorBuilder는 다양한 종류의 바이러스를 생산하는데 많은 전문성을 보유하고 있습니다. 유전자 치료제 개발 파이프라인과 함께 전반적인 수요를 충족시키기 위해 다양한 규모와 품질의 바이러스를 공급하고 있습니다. 아데노부속바이러스 (AAV) 및 렌티바이러스의 대규모 GMP 제조를 위한 플랫폼 기술을 확립하고 검증하였습니다. 아데노바이러스, MMLV, 헤르페스단순포진바이러스 (HSV) 및 수포성구내염바이러스 (VSV) 등 다른 종류의 바이러스 벡터도 생산하고 있습니다. 신약 탐색 연구, 전임상 연구, 임상 시험 및 상용화 등 다양한 목적에 부합하는 여러 등급의 바이러스를 생산할 수 있습니다.
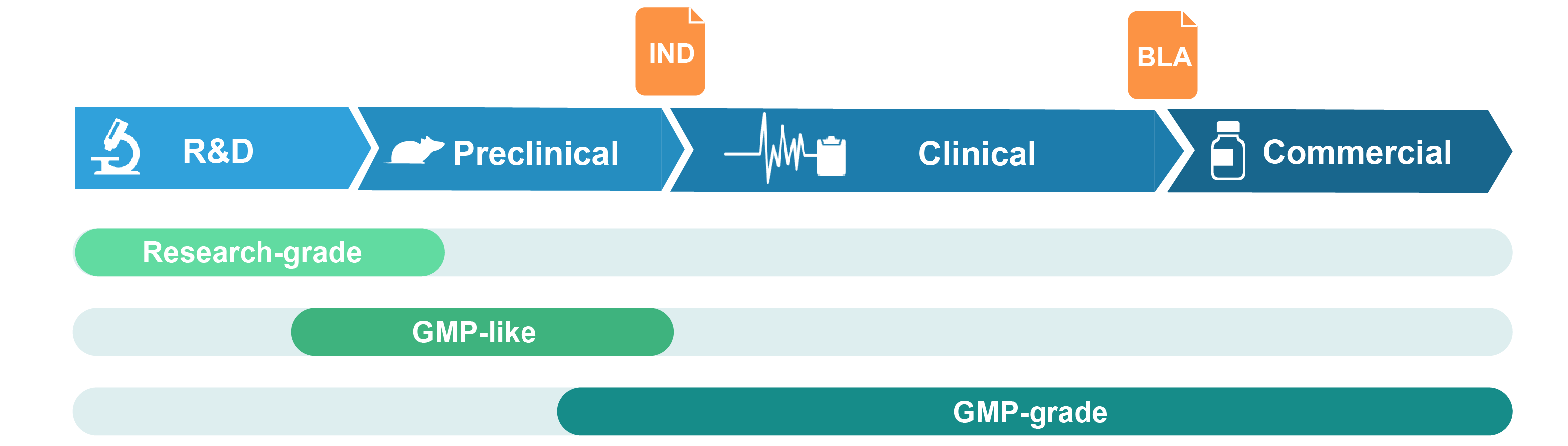
다양한 등급의 바이러스 제공
-
Research-grade virus
Research-grade 바이러스는 기초 연구와 신약 탐색 연구에 적합합니다. 일반적인 세포 배양 시설에서 생산됩니다. 고객이 요구하는 기준에 부합하는 바이러스 품질을 보장하기 위해 엄격한 QC assay들을 수행합니다.
Visit here to learn more about our research-grade virus -
GMP-like virus
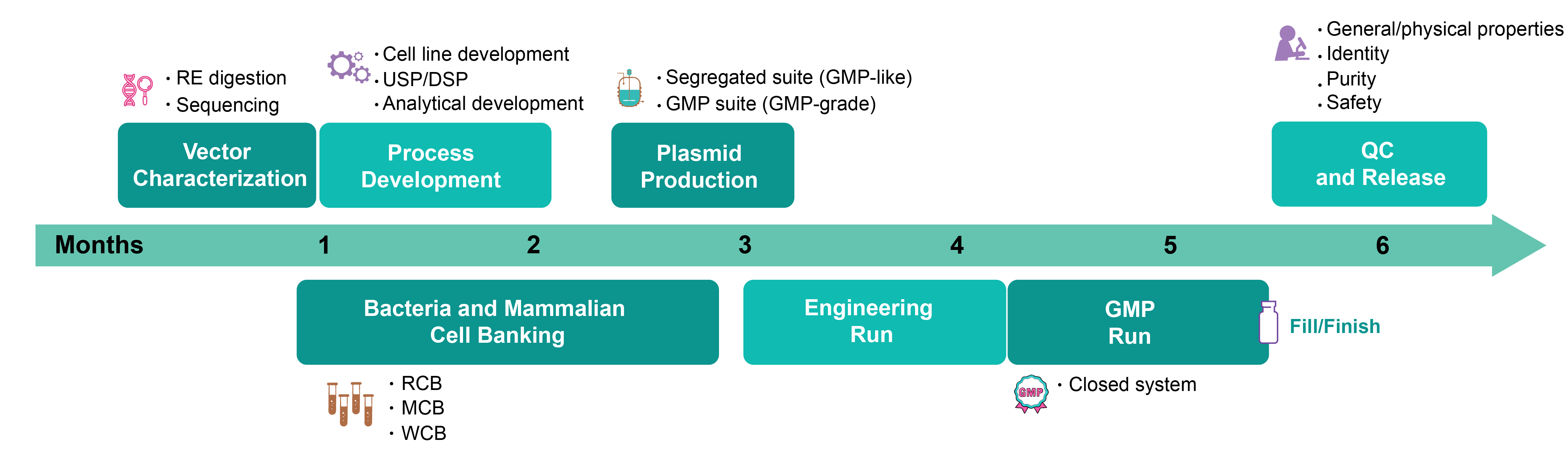
GMP-like 바이러스는 약물 안전성 및 대사에 대한 동물실험과 같은 전임상 연구에 적합합니다. 생산 공정 및 품질관리를 문서 관리와 추적성을 갖춘 GMP 수준 가이드라인의 주요 특징에 따르는 방식으로 생산됩니다. 따라서 GMP-like grade는 최종 GMP 제품을 소규모로 유사한 방식으로 생산하는 것이라고 볼 수 있으나, 비용이 매우 낮고 훨씬 빠릅니다. 제품 출고시에 COA(certificate of analysis)가 제공됩니다. TSE/BSE statement도 요청시 제공될 수 있습니다.
-
GMP virus
GMP-grade 바이러스는 GMP 가이드라인을 엄격히 준수하는 공인된 GMP 세포 배양 시설에서 생산됩니다. 생산 공정 전반에 걸쳐 종합적인 품질 보증 시스템이 적용되며, 바이러스의 품질과 안정성이 고객이 원하는 규격과 기본 규정을 충족하는지 보증하기 위해 광범위한 in-process 및 release QC assay들을 수행합니다. 제품 출고시에 생산 공정을 완전히 문서화한 batch release 보고서와 COA가 제공됩니다.
Comparison of different grades of virus
| Research-grade | GMP-like | GMP-grade | |
|---|---|---|---|
| Applications | Basic research, drug discovery, and preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism | Preclinical studies, clinical studies, and commercialization |
| Production scales | 5x1010 to 1014 GC per batch | 5x1013 to 1017 GC per batch | 1014 to 1017 GC per batch |
| Turnaround time | 10-50 days | 4-5 months | 6-12 months |
| Production site | In parallel production in standard BSL-2 laboratory | Produced in segregated BSL-2 production suites | Produced in certified GMP suites (BSL-2) |
| Quality system | ISO9001 | ISO9001 while adopting key features of GMP manufacturing | ICH quality guidelines for GMP manufacturing |
| Document control and traceability | No | Yes | Full traceability |
| Vector characterization | RE digestion, ITR sequencing | Sequencing | Sequencing |
| Process development | No | Performed on a case-by-case basis depending on individual project needs | Yes |
| Cell banking | No | Available upon request | Yes |
| Antibiotic-free | No | Available upon request | Yes |
| Animal component-free | No | Available upon request for suspension culture | For suspension culture |
| Purification | Ultracentrifugation | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography |
| QC and release tests | Titer measurement, SDS-PAGE, endotoxin detection, sterility testing, mycoplasma detection, etc. | Performed on a case-by-case basis depending on individual project needs | Full panel QC assays, analytical development upon individual project needs |
| Aseptic fill/finish | No | Available upon request | Yes |
| Storage of retain sample | Available upon request | Available upon request | Yes |
| Other deliverable | COA | 1. COA 2. Manufacturing summary 3. TSE/BSE statement upon request |
1. COA 2. TSE/BSE statement 3. CTD documents 4. Others (BMR etc.) upon request |
| Research-grade | GMP-like | GMP-grade | |
|---|---|---|---|
| Applications | Basic research, drug discovery, preclinical studies | Preclinical studies such as animal testing of drug safety and metabolism | Preclinical studies, clinical studies, and commercialization |
| Production scales | >2.5x107 TU | 109 to 1012 TU per batch | 5x109 to 1012 TU per batch |
| Turnaround time | 8-16 days | 4-5 months | 6-12 months |
| Production site | In parallel production in standard BSL-2 laboratory | Produced in segregated BSL-2 production suites | Produced in certified GMP suites (BSL-2) |
| Quality system | ISO9001 | ISO9001 while adopting key features of GMP manufacturing | ICH quality guidelines for GMP manufacturing |
| Document control and traceability | No | Yes | Full traceability |
| Vector characterization | No | Sequencing | Sequencing |
| Process development | No | Performed on a case-by-case basis depending on individual project needs | Yes |
| Cell banking | No | Available upon request | Yes |
| Antibiotic-free | No | Available upon request | Yes |
| Animal component-free | No | Available upon request for suspension culture | For suspension culture |
| Purification | Ultracentrifugation | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography | Ultracentrifugation, affinity purification, IEX purification, mixed-mode chromatography |
| QC and release tests | Titer measurement, sterility testing, mycoplasma detection, etc. | Performed on a case-by-case basis depending on individual project needs | Full panel QC assays, analytical development upon individual project needs |
| Aseptic fill/finish | No | Available upon request | Yes |
| Storage of retain sample | Available upon request | Available upon request | Yes |
| Other deliverable | COA | 1. COA 2. Manufacturing summary 3. TSE/BSE statement upon request |
1. COA 2. TSE/BSE statement 3. CTD documents 4. Others (BMR etc.) upon request |
Platform Technologies
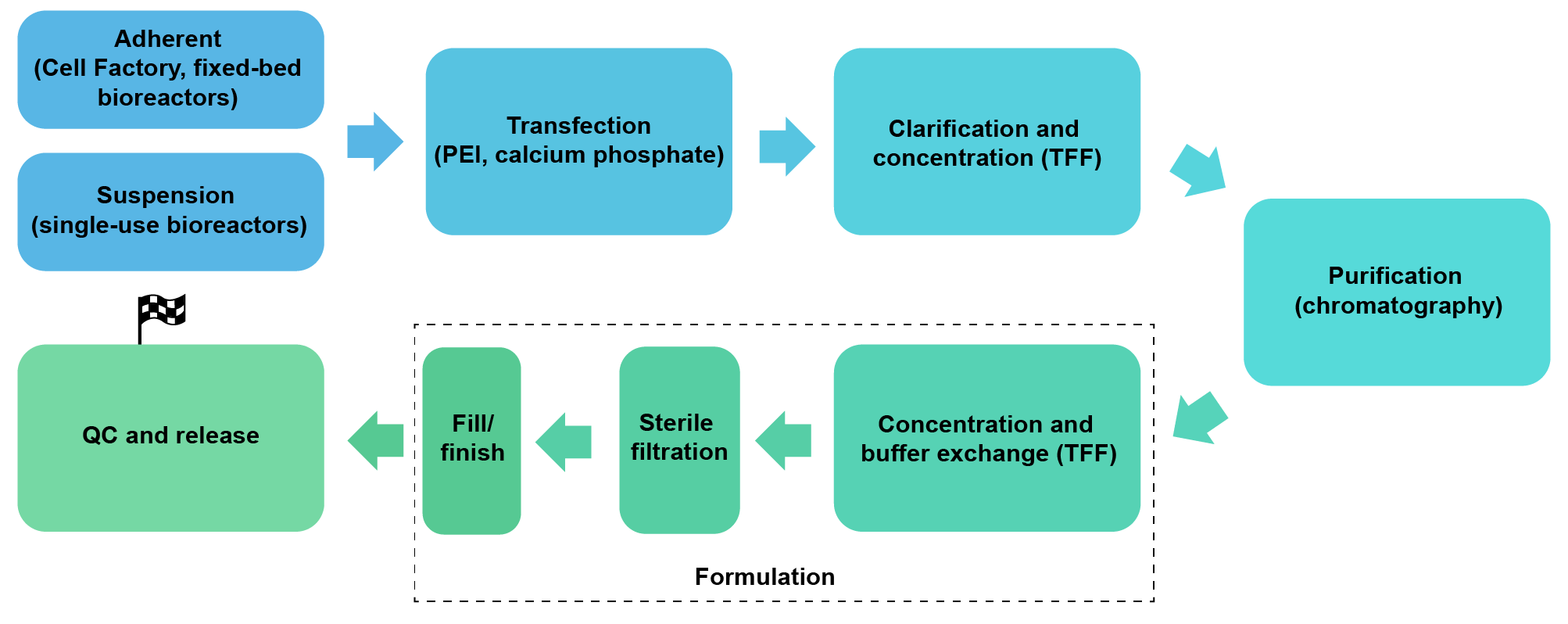
We package AAV in HEK293 cells under either adherent conditions (Cell Factory or fixed-bed bioreactors) or serum-free suspension conditions (up to 200 L single-use bioreactors). We also package AAV in suspension Sf9 insect cells. We can achieve a scale of up to 1017 GC AAV per batch.
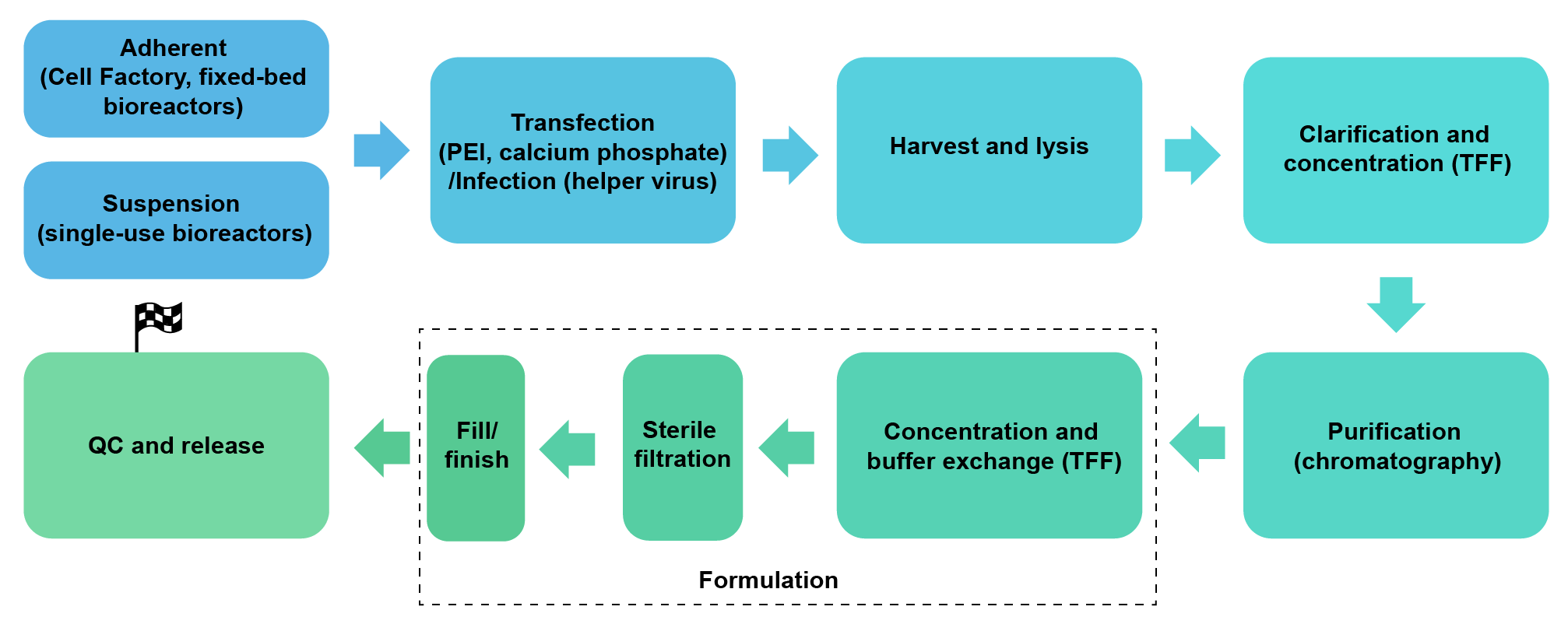
We package lentivirus (2nd and 3rd generation, pseudotyped with VSV-G or other viral surface proteins) in HEK293, under either adherent growth conditions (Cell Factory or fixed-bed bioreactors) or serum-free suspension conditions (up to 200 L single-use bioreactors). We can achieve a scale of up to 1012 TU per batch.